PORTLAND, Maine — Winter in Maine comes with a few predictable things: snow, ice, and cold temperatures. We normally deal with slick roadways by putting down salt to keep ice from forming. It's been especially cold here recently and after a certain point the cold makes the salt much less effective, but why?
To start off, let’s talk about the molecular structure of water. When in its liquid state, the molecules are loosely packed and are more free flowing.

As the water gets colder and begins to freeze, the molecules become more tightly packed forming tighter bonds. That’s when we put road salt down. Salt disrupts the freezing process and prevents those tighter bonds from forming.
In this example, the yellow circles represent the salt. They are disrupting that freezing process and lowing the freezing point of the water.


This allows the salt/water to stay in a liquid form. This is true a good portion of the winter when temperatures are closer to the freezing point. When the temperature is 32 degrees, road salt is very effective at melting snow and ice. When we get much colder, things start to change.


As a general rule of thumb, when the temperature reaches 15 degrees or less, salt is less effective. This is because the freezing point is lowered, not eliminated. The point when the salt/water combination goes from a liquid to a solid is around 15 degrees...which means we would still have ice on the roads. At that point, the amount of salt needed to melt all of the ice would be impractical to use.


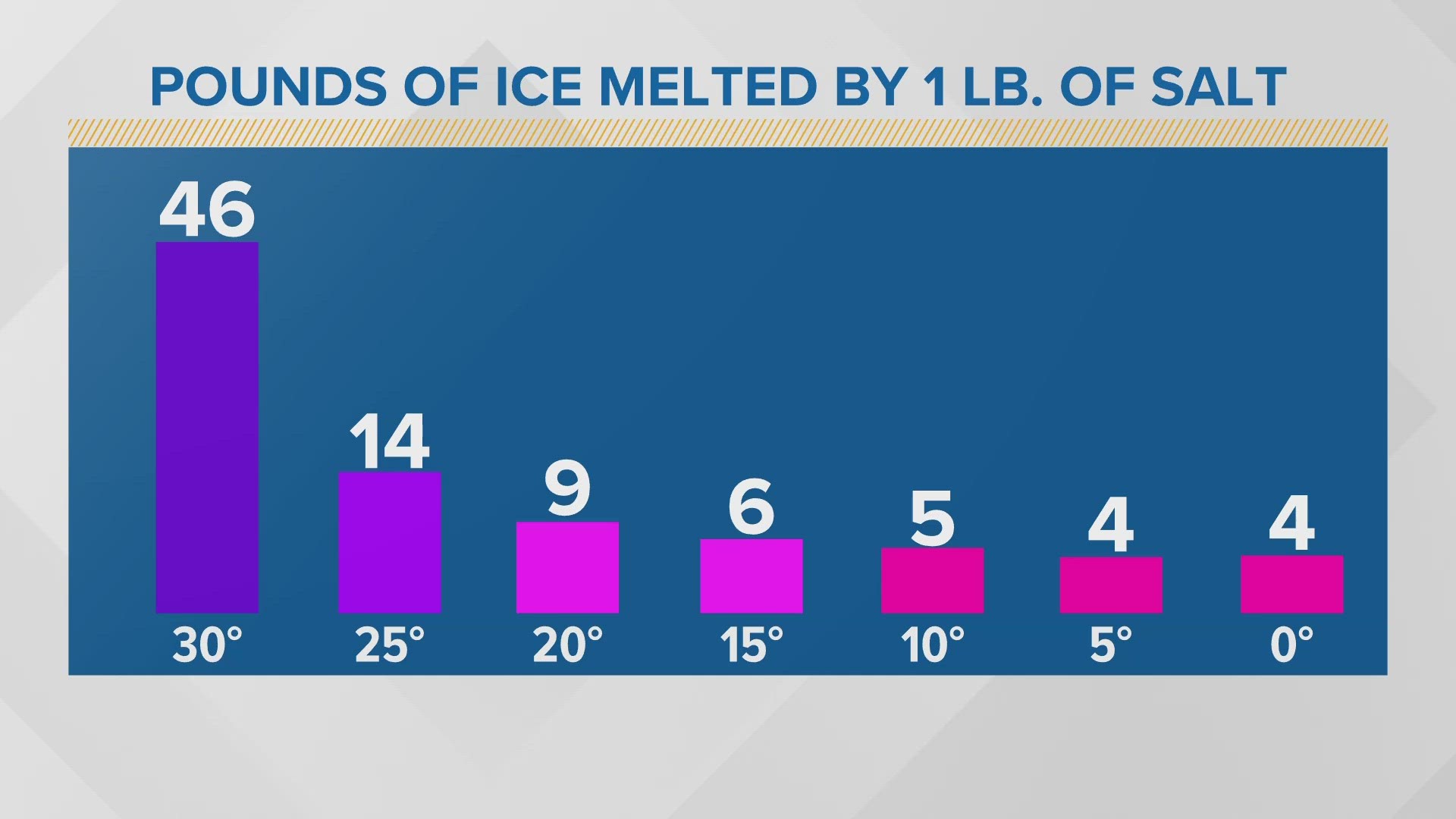
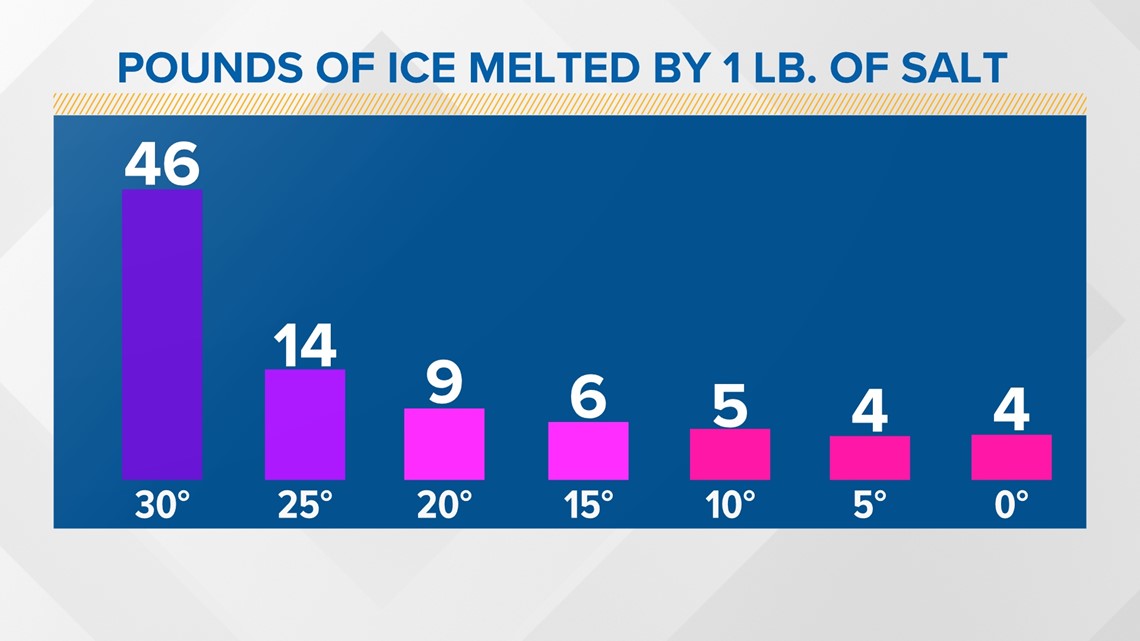
You can melt 46 pounds of ice with a pound of salt when the temperature is 30 degrees. Once we cool off just five degrees, that amount dramatically decreases, and we only need more salt from there.

That's why, when it’s very cold, we begin to throw down sand in Maine. It doesn't melt the ice, but it still gives your tires some traction.
Be careful with these colder mornings because we could see more black ice on the roads as the salt struggles to melt it.