POLAND and LEWISTON, Maine (NEWS CENTER) - Two women from Maine and thousands across the country are blaming a popular flea and tick medication for making their dogs sick - and worse.
Both the company that makes the drug, and some local vets say there is no scientific evidence that supports these claims.
It's a real debate between pet owners and veterinarians.
NOW spoke with two women, one from Poland and one from Lewiston.


They have two different dog breeds, and they've never even met - but their experiences are very similar.
“Vomiting...the dehydration...lot of drinking, just excessive amount of drinking, the diarrhea started the next day, the fatigue, still very lethargic, the trembling all over,” Barbara Mandy of Lewiston and Ashley Jardine of Poland listed nearly identical symptoms.
It's a story of two women and their dogs, from two Maine towns, two years apart – but with one very similar story.


“We don't have kids,” Ashley said. “Like, human children. These dogs are our kids.”
“The minute I saw [Callie], it was just instant love,” Barbara of Lewiston added. “She went everywhere with me. Just was my constant companion.”
Companions through thick and thin.
Callie got sick in September 2015; Lizzie earlier in October.
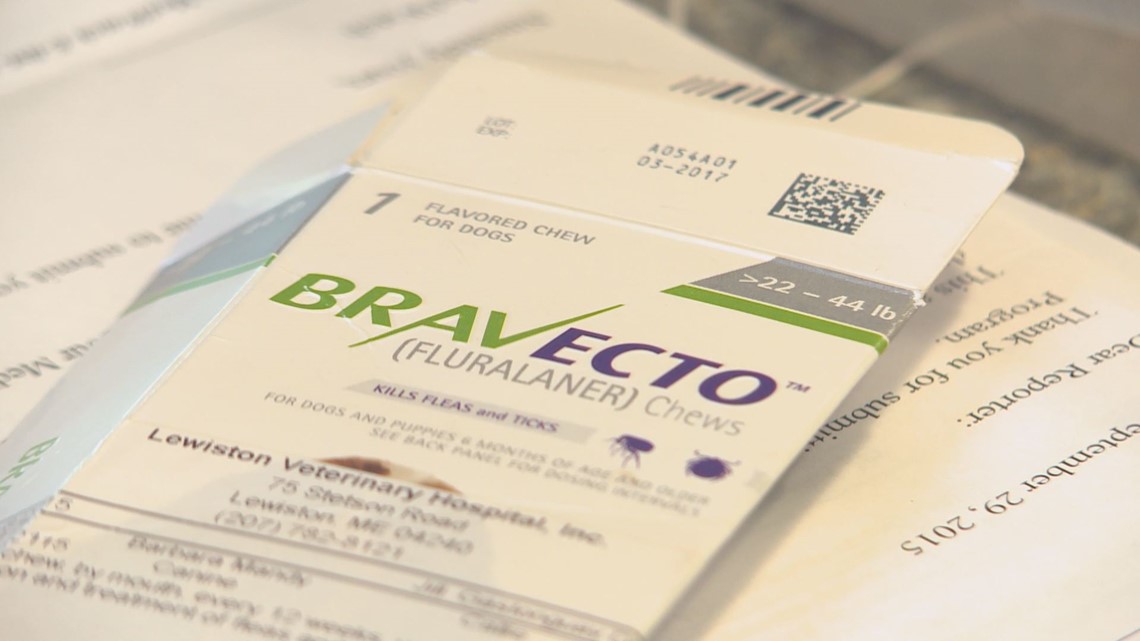
Both owners say the symptoms started within minutes of taking BRAVECTO, a prescribed, ingestible flea & tick medication.

Ashley explained the moments after she gave the chew to her dog, Lizzie. “I got nervous, and my immediate thought was, 'Okay, she's experiencing some side effects from this medication I just gave her.'”
Lizzie became lethargic, and started vomiting.
Callie passed out and was unresponsive for 10 minutes.
“[It was] panic,” said Barbara. “Panic. Just crying. Panic. Shaking her. Crying, 'Callie, Callie, Callie.'”


Merck Animal Health released BRAVECTO in 2014.
According to the company, it has been proven safe in more than 55 research studies.
Ashley said she wished she “had done [her] homework before this product.” That homework included a quick internet search, and connecting with a Facebook group – 40,000 strong - "to talk about BRAVECTO and any potential impact it had on your dog."
BRAVECTO'S packaging says common side effects are nausea, diarrhea, and loss of appetite.
But thousands of people claim the effects are much worse. In response to an open records request, NEWS CENTER learned that since BRAVECTO's release in 2014, more than 16,000 complaints (16,459) have been filed with the FDA about adverse effects in dogs who took the chew. That's compared to 3 complaints for dogs who used the topical version of BRAVECTO. The reports for the chew cite seizures (508), vomiting (7,715), and even death (499).
BRAVECTO complaints to FDA by NEWSCENTER on Scribd
Ashley took 9-year-old Lizzie, who is diabetic, to see 4 vets—each one said BRAVECTO isn't the problem.
Nursing Lizzie back to health has cost more than 4 thousand dollars.



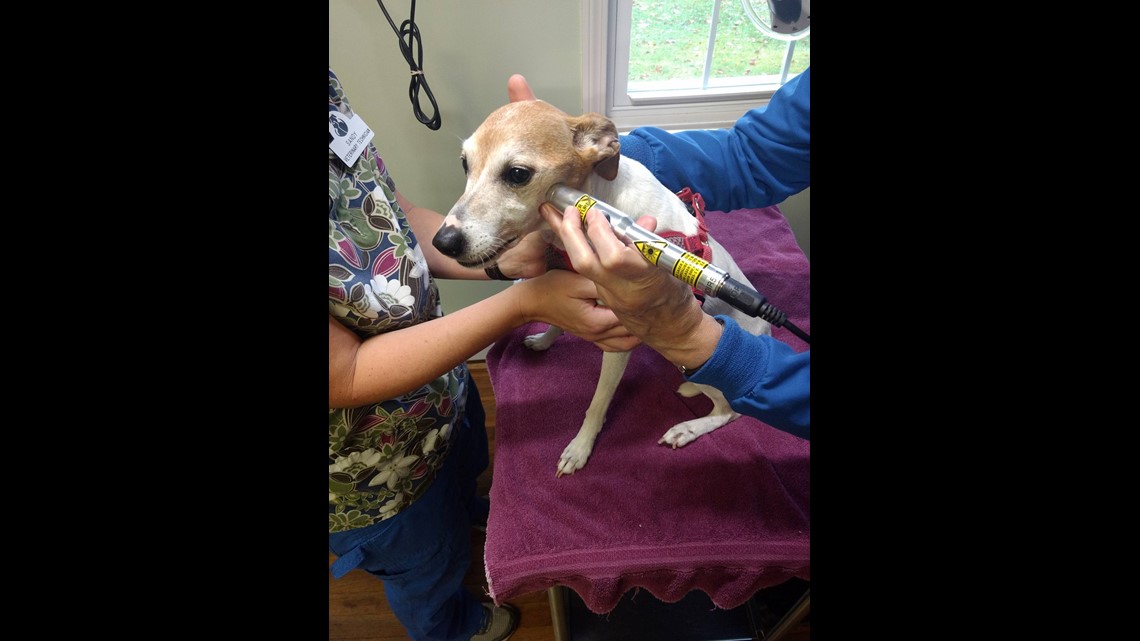
Barbara says she went to the vet in 2015 when she needed a quick flea and tick medication for Callie before visitng her grandchildren. “They assured me that [BRAVECTO] was safe,” she explained. Her 8-month old Australian labradoodle, Callie, was prescribed BRAVECTO. “I remember asking three times, 'Are you sure it's safe?' Because there was just a little voice that said, 'ingestible pesticide,’” she said.
Merck Animal Health denies that BRAVECTO caused these illnesses. A spokesperson, Noreen Verbrugge, said that in Lizzie's case, "It would be premature and irresponsible - and potentially actionably false - to suggest or speculate otherwise."
No veterinarian has been able to definitively determine that BRAVECTO either was or was not the cause of illness for both Lizzie and Callie.
“You want to trust these doctors,” Ashley said. “You want to trust in these pharmeceutical companies.”
Why no scientific evidence? Vets we spoke with say many people don't get necropsies performed to determine their pet's cause of death.
This was true for Barbara. “Emotionally, I couldn't let them do [the necropsy],” she said. “I couldn't do it. And it was probably a mistake.”


Both women have filed complaints of adverse effects with the FDA and to Merck Animal Health. Barbara also filed with the SPCA Poison Control Center and the European Medicines Agency.
In an email to NEWS CENTER, a Merck representative wrote that the company takes "every report of a potential adverse event seriously and investigate[s] thoroughly."
In both cases - Merck has paid some of the vet bills.
Ashley says two weeks later, Lizzie still struggles with jaw paralysis and vomiting - but she's slowly making a recovery.
Callie wasn't as lucky.
Barbara documented every symptom Callie experienced, as her health declined over the course of seven weeks. She died seven weeks and two days after her dose of BRAVECTO.
It took Barbara almost two years to have the courage to bring a new dog into the family – and even with Sunny Grace around, she can't get Callie off her mind.


“I will always blame myself,” Barbara said. “That guilt will never go away, that I gave that [chew] to that poor sweet dog.”
What both Ashley and Barbara want is more testing done, and a more descriptive warning label on BRAVECTO.
But if there's no scientific evidence that BRAVECTO caused these symptoms, how – and why – would the company put a stronger warning on the box?
Vets say not enough research has been done on dogs whose owners reported problems with the treatment. It's hard to determine a link between BRAVECTO and the symptoms their pets are experiencing. So, more necropolises would have to be done and tested before the company could consider changing the warning label.
What has your experience been with BRAVECTO? Email asknow@wcsh6.com or asknow@wlbz2.com to let us know.

