PORTLAND, Maine — As surging cases of COVID continue to put pressure on Maine hospitals, a new device could soon help expand much-needed capacity.
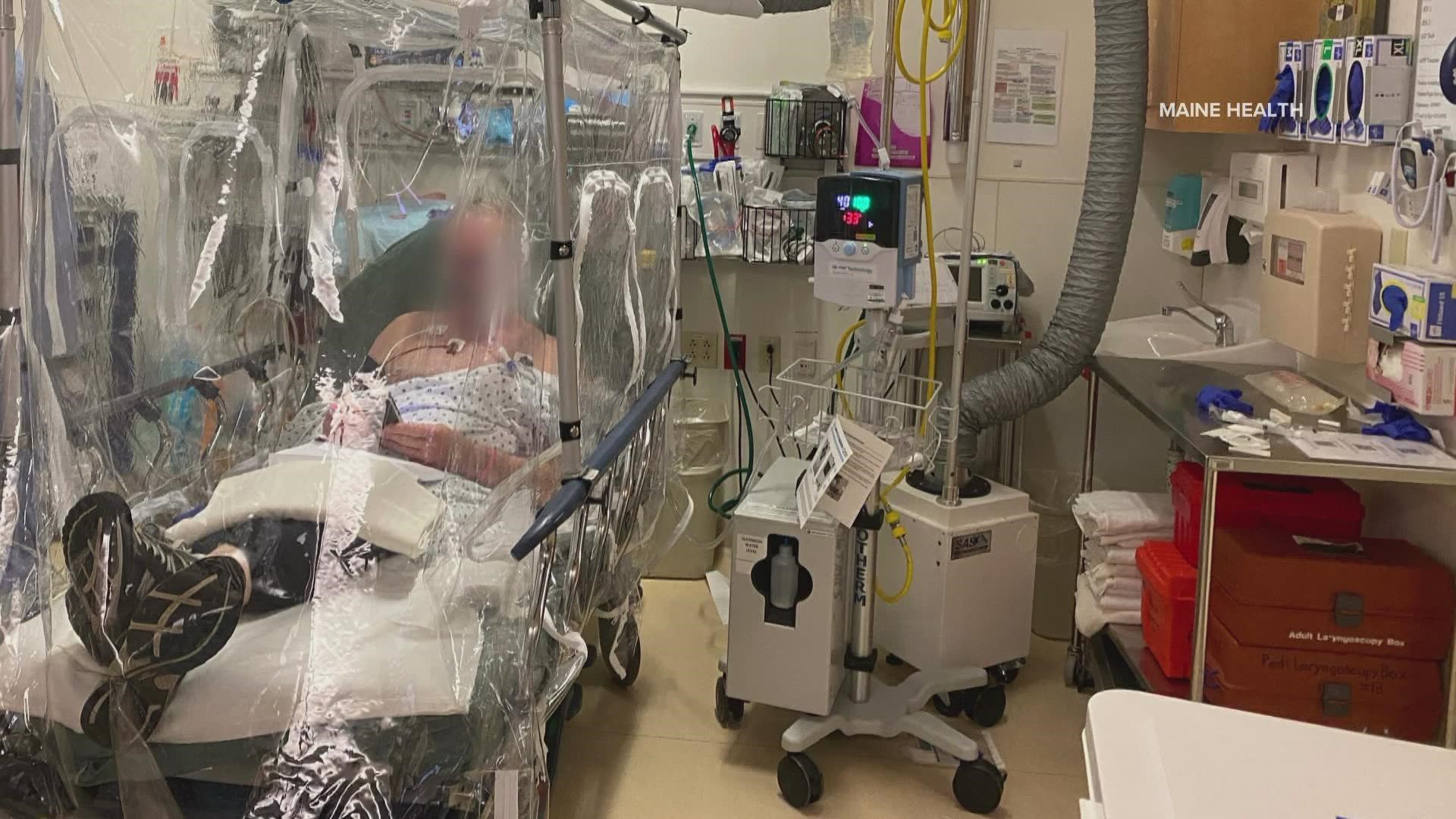
Maine medical doctors and medical equipment companies are developing a tent-like device that converts a hospital stretcher into a negative pressure chamber. Like a negative pressure room, it's designed to contain contagious viruses like COVID from spreading.
The Collapsible Aerolite Particle Enclosure (CAPE) can be used to contain infectious viruses and one day could be deployed to medical facilities not only in Maine but across the country.
Patient trials are currently underway at Maine Medical Center for the new device that could be a game-changer for hospitals squeezed by an onslaught of COVID cases.
"If you have two patients in a room, and one tests positive, and you don't have a place to put that patient, this device can be deployed immediately to isolate that patient," Dr. Samir Haydar said.
Haydar is an emergency physician at Maine Medical Center. He is part of a team developing the CAPE device.
"If you have an infectious agent inside this device, it's going to stay in this device," Haydar said.
Two emergency room residents at Maine Medical Center initially came up with the idea at the beginning of the pandemic, using materials from a hardware store. Doctors began collaborating with two Maine companies, the Baker Company and Thermoformed Plastics of New England, to develop a prototype.
David Eagleson, CEO of the Baker Company, based in Sanford, showed NEWS CENTER Maine how the filtration system pulls air out of the CAPE.
"You can see the smoke now with the side port open is being pulled into the enclosure, rather than being pushed out," Eagleson said.
The company manufactures microbiological safety cabinets for research labs and ventilation systems for medical facilities.
Medical staff, meanwhile, can access a patient inside the chamber through a range of different sized openings, from putting in an IV or hooking up a patient to a ventilator. The CAPE can also be set up in just minutes and moved to other floors throughout the hospital.
"They can be ready at the emergency department when you walk in. And say you don't feel good, and they say get in the tent, and they can start monitoring you," Paul Tyson, of Thermoformed Plastics of New England, said.
The Biddeford-based company makes FDA-approved packaging shipped across the world and helped design the prototype. Funding from the MaineHealth Innovation Bonfire Fund allows the team to focus on securing emergency use authorization approval from the Food and Drug Administration.
Meanwhile, the CAPE device continues to be tested on patients, and so far, the feedback has been positive. A decision by the FDA is expected within the next month or so.