SCARBOROUGH, Maine — Abbott, a global health care company that focuses on point-of-care testing and technology, announced Friday that the U.S. Food and Drug Administration (FDA) has issued Emergency Use Authorization (EUA) for a COVID-19 test that delivers results in as little as five minutes. Abbott has a manufacturing facility in Scarborough, and a company spokesperson confirmed with NEWS CENTER Maine the tests would be made in Maine.
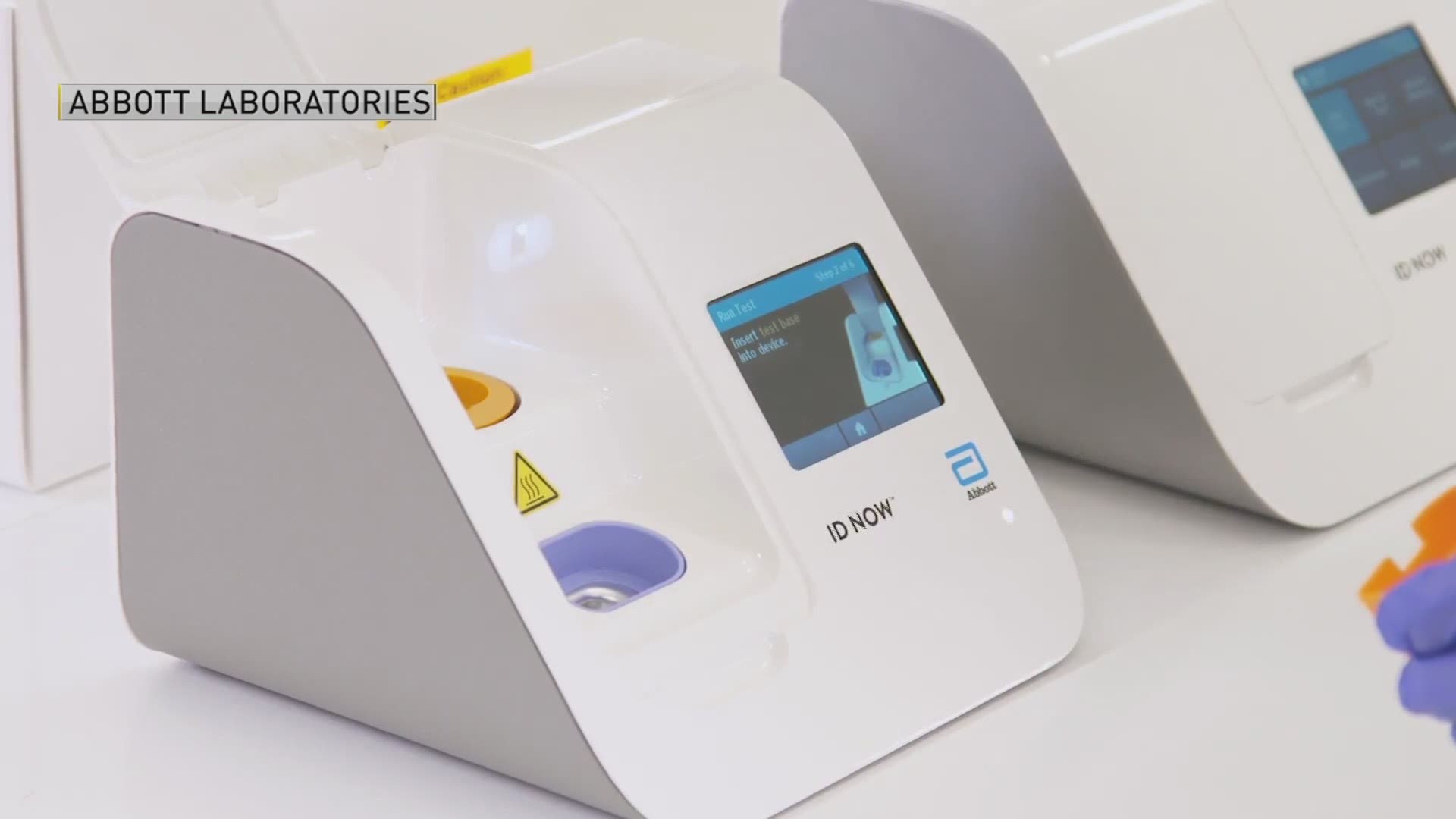
Abbott says the test is the fastest available molecular point-of-care test for the detection of novel coronavirus (COVID-19) and can deliver positive test results in as little as five minutes, and negative results in 13 minutes. The test will run on the company’s ID NOW platform, a portable instrument that can provide fast results in a wide range of health care settings, such as doctor’s offices, urgent care clinics, hospitals, and emergency rooms.
"The COVID-19 pandemic will be fought on multiple fronts, and a portable molecular test that offers results in minutes adds to the broad range of diagnostic solutions needed to combat this virus," Robert B. Ford, president and chief operating officer of Abbott, said. "With rapid testing on ID NOW, healthcare providers can perform molecular point-of-care testing outside the traditional four walls of a hospital in outbreak hotspots."
Abbott hopes to have the ID NOW COVID-19 tests available next week and expects to ramp up manufacturing to be able to deliver 50,000 tests per day, according to a press release.
This is the company’s second test to receive Emergency Use Authorization by the FDA for COVID-19 detection, but the first that will be made at the company’s Maine facility. The other test is Abbott’s m2000 RealTime SARS-Cov-2 test. Combined, the company expects to produce about 5 million tests per month.


The ID NOW test is small, lightweight—weighing only 6.6 pounds—and is portable; it’s about the size of a toaster, Abbott says. The test uses molecular technology, which is valued for its high degree of accuracy.
The company is working with the FDA to deploy tests to areas where they can have the greatest impact.
RELATED: Maine company makes COVID-19 swabs
At NEWS CENTER Maine, we’re focusing our news coverage on the facts and not the fear around the illness. To see our full coverage, visit our coronavirus section, here: /coronavirus
NEWS CENTER Maine Coronavirus Coverage
RELATED: Preble Street and USM partner to provide 50-bed homeless shelter during coronavirus pandemic
NEWS CENTER Maine YouTube Coronavirus Playlist